Scientific Cores
Cell Separation and Culture (CSC) Core
The Cell Separation and Culture Core provides vital services to Center members.
First, it provides services that are frequently utilized by numerous funded investigators, thereby allowing sharing of resources and cost reduction. Second, it fosters an environment for collaboration and growth of investigators. Third, it provides a mechanism for development of new techniques that will benefit the investigators.
Goals of the CSC Core are to:
- Provide high quality, consistent services needed by a large number of independently funded investigators to improve research efficiency by sharing resources and lowering costs.
- Foster an environment for interdisciplinary collaboration and growth.
- Train investigators at USC and elsewhere in techniques for cell isolation and separation.
- Develop new services that the funded investigators and junior faculty will likely use in the future.
Director: Takeshi Saito, M.D., Ph.D.
As the new Director of this core, Dr. Saito supervises the work of the Core and oversees the utilization of the Core and the development of new services. He will be responsible for quality control of isolated cells, and cell line repository.
Co-Director: Lucy Golden-Mason, Ph.D.
Dr. Golden-Mason has extensive experience in translational science and of a broad array of cell separation and culture techniques. She will provide support to members in the form of experimental design and data analysis. She also manages the expansion of the Core in NRT 4514 with a new BSL2 BioSafety Cabinet, BD FACSAria Fusion Flow Cytometer/Sorter, and 10X Chromium Controller for single-cell RNA sequencing.
Core Research Associate: Lina He, M.D.
Dr. He provides the 20 years of experience in tissue culture and provides the services of this core.
Facilities and Resources
Location: HMR 803
Services:
Cell Culture component of the Core:
The Cell Culture component of the Core provides fee for services.
Existing Services:
Provides isolation and culturing of rat and mouse hepatocytes from normal and diseased livers, and cell line banking. Provides training to investigators at USC or outside of USC that are NIH-funded.
Cell Separation component of the Core:
The Cell Separation component of the Core provides assistance to Center members in the use of Core’s following equipment to isolate cell subtypes and analyze their function. There is currently no fee to use these instruments and Dr. Golden-Mason is in charge of training users and providing assistance when needed.
- BD FACSVerse Flow Cytometer (HMR-803)
- Miltenyi Biotec AutoMACS Pro Separator (HMR-803)
- VWR Symphony CO2 Incubator for Core Cell Cultures (HMR-804)
- Sanyo IncuSafe MCO-18A1C CO2 Incubator for RCLD members’ shared use (HMR-804)
- Two VWR 35VHC Liquid Nitrogen Storage Tanks (HMR-804)
- Nikon Diaphot microscope (804)
- LabConco Model 3440809 BioSafety Cabinet (HMR-804B)
- Chromium Controller for single-cell RNA sequencing (NRT-4514)
- MidiMACS™ Separator (NRT-4514)
- OctoMACS™ Separator (for 8 columns) (NRT-4514)
- Motic light microscope with hemocytometer (NRT-4514)
- Nikon Alphaphot YS Microscope (NRT-4514)
- Countess II Automated Cell Counter (NRT-4514)
- Two Forma Series II CO2 Incubators (NRT-4514)
- Fisher Scientific Vortex Mixer (NRT-4514)
- Forma Class II, A2 BioSafety Cabinet to accommodate the BD FACSAria™ Fusion flow cytometer/sorter for the BSL2 operations (NRT-4514D)
- BD FACSAria™ Fusion flow cytometer/sorter (NRT-4514D)
- Beckman Coulter Allegra X-15R Centrifuge (NRT-4514E)
Costs:
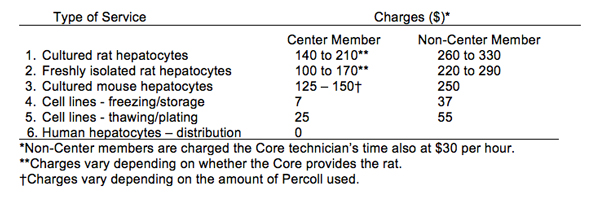
There are charges for most of the services provided by the Culture component of the Core (see Table 1) to cover the cost of animals and basic supplies. For Center members the fee includes reimbursement of supplies and animal cost. For non-Center members, the fee also includes labor. When a cell preparation is shared by multiple investigators, the cost to the investigator is adjusted accordingly. Currently, there is no charge for use of the Cell Separation equipment. However, this may change depending on future budgetary constraints, so that a chargeback system may be necessary to partially cover service contracts of equipment.
Table 1. Summary of Charges*
**Please acknowledge the Liver Center in your abstracts and publications
- Citation of the Liver Center as a professional affiliation
- Acknowledgement of specific Liver Center support where appropriate (core use, pilot funding)
- Link all liver-related publications to DK048522 in PMCID
Cell and Tissue Imaging (CTI) Core
The Core offers assisted imaging services, using confocal and fluorescence microscopy primarily with its Leica SP8 Confocal and Nikon TE-300 Diaphot systems as needed by Center members.
Goals of the CTI Core are to:
- Maintain the confocal and fluorescence microscopy facilities of RCLD.
- Provide assisted confocal and fluorescence microscopy and image processing to Center members.
- Provide education and training in diverse microscopy applications supported by the Core.
- Provide access and financial support for RCLD Center members to access USC Core imaging facilities enabling 3D-SIM, spinning disk confocal microscopy and multiphoton microscopy
Director: Kinji Asahina, Ph.D.
Dr. Asahina is the new Director of this core. He will supervise the work of the Core will oversee the scientific direction and development of new applications and methodologies.
Associate Director: Lucy Golden-Mason, Ph.D.
Dr. Golden-Mason has extensive experience in translation science and of cell imaging techniques. She will assist Dr. Asahina in overseeing the Core.
Associate Director: Janos Peti-Peterdi, M.D.
Dr. Peti-Peterdi provides assistance and expertise with the Muti-photon system and will provide training and access to his Multi-Photon Microscopy Core.
Core Manager: Heather Johnson, B.A., M.S.
Ms. Johnson supervises the use of the Zeiss LSM 510 and Nikon imaging systems, providing assisted imaging and training.
Facilities and Resources
Locations: HMR 610(C), HMR 615, and NRT 4515(E)
Services:
Dedicated Core Equipment:
- Leica SP8 with HyVolution confocal microscopy imaging system (HMR 610C)
- Nikon TE-300 Diaphot imaging system for fluorescence and DIC (HMR 610C)
- Imaging workstation (HMR 615)
- IncuCyte Live Cell Imaging and Analysis System (NRT 4515E)
**Instrumentation facilitated/subsidized for access by Center members (upon successful competitive renewal of RCLD grant):
- GE DeltaVision OMX system:
http://cemma.usc.edu/instruments/ - Leica TCS SP5 Multiphoton system:
- Perkin Elmer Ultraviewers 6 line Spinning Disk Laser Confocal Microscope:
http://uscnorriscancer.usc.edu/core/confocal/
Costs:
These chargebacks are implemented on a two-tiered basis for the combined hourly usages of both systems, as follows:
Members: $25/hr with a total annual cap of $650
Non-members: $35/hr with a total annual cap of $1,300
* For sign up and access to the Core contact Ms. Heather Johnson (heather.johnson@usc.edu)
**The pilot funds available for investigator access to the new imaging facilities described above are provided by the School of Medicine with an annual contribution of $50,000.
**Please acknowledge the Liver Center in your abstracts and publications
- Citation of the Liver Center as a professional affiliation
- Acknowledgement of specific Liver Center support where appropriate (core use, pilot funding)
- Link all liver-related publications to DK048522 in PMCID
Liver Histology (LH) Core
The Liver Histology Cores provides distinct services with….
Goals of the LH Core are to:
- Perform routine histology, i.e. regular paraffin processing, sectioning and H&E, Sirius red and Oil red staining and provide assistance in interpretation and quantitation of findings
- Cut unstained slides, which the users can subsequently stain themselves
- Maintain a deparaffination and dehydration line of chemicals under a hood, accessible to users
- Provide frozen sectioning
- Add other services, based on the evolving needs of our user base (e.g. due to demand, over time the Core has added TUNEL, caspase 3, PCNA, BrdU, Grp78, Cox IV, F4/80, myeloperoxidase and Ki67 stainings
- Provide and train investigators in the use of a state-of-the-art laser micro-dissection system and customized slide preparations
- Provide a stand-alone workstation with microscope, image capture camera and processing software for independent and/or assisted use and analyses-interpretation of histology image
- Assist investigators in development of immunohistochemistry for specific needs
- Assist investigators in interpretation of histological findings and in the development of some quantitative and semi-quantitative systems for scoring findings (e.g. necrosis, inflammation, fibrosis, steatosis)
Director: Gary C. Kanel, M.D.
Dr. Kanel provides extensive experience in the technical aspects of the preparation, staining and immunohistochemistry of liver. He supervises the Core, assess the quality of the slide preparation, assists in troubleshooting and is available on a scheduled basis or by appointment to meet and review slides with Center members.
Core Manager: Heather Johnson, B.A., M.S.
Ms. Johnson provides support in all aspects of the Core’s services, accounting and billing. She also teaches and assists investigators in the usage and applications of the core’s LMD7000 laser micro-dissection system and Nikon Eclipse 80i Microscope image capture and processing system.
Core Research Specialist: Lina He, B.S.
Ms. He has over 20 years of laboratory and histology experience. She will provide a new service for RCLD members: extraction of high quality DNA/RNA from formalin-fixed paraffin-embedded (FFPE) tissue.
Facilities and Resources
Location: HMR 610 (A), 615
Equipment:
- TBS ATP-120 Automatic Tissue Processor (HMR-610A)
- TBS Tissue Embedding Center (HMR-610A)
- Leica Autostainer XL (HMR-610A)
- Sakura Accu-cut SRM Microtome (HMR-610A)
- Leica Bond Max Immunostainer (HMR-610)
- Microm HM550 VP Cryostat (HMR-610)
- Leica LMD7000 laser micro-dissection system, complete with all modules, components and set of lenses (HMR-615).
- Nikon 80i Eclipse microscope with Optronics MacroFire True Color 4MP (2048 x 2048P) digital CCD camera w/PictureFrame software (HMR-615).
Costs:
The fee schedule for our current menu of services appears on the Request for Histology Services form.
*To access and use the Core contact Ms. Heather Johnson (heather.johnson@usc.edu). Users are required to fill in and submit the Request for Histology Services form, along with delivering their tissue specimens to the Core at HMR-610A.
**Please acknowledge the Liver Center in your abstracts and publications
- Citation of the Liver Center as a professional affiliation
- Acknowledgement of specific Liver Center support where appropriate (core use, pilot funding)
- Link all liver-related publications to DK048522 in PMCID
Analytical-Metabolic-Instrumentation (AMI) Core
The AMI Core is an essential resource for a large number of Center members and their labs, offering critically needed analytical services, provided through a versatile multi-detection HPLC system, plus an extensive base of common as well as advanced, state-of-the-art resources for shared access/use.
Goals of the AMI Core are to:
- An extensive base of critically needed common and advanced equipment for shared access-use
- Assisting-advising investigators and their personnel in evaluation, selection and optimal utilization of the Core’s base of resources and services
- Training-orientation of a constantly evolving base of users in hands-on operation of the Core’s resources
- A versatile menu of HPLC analyses and applications, using multiple detection methodologies
Director: Lucy Golden-Mason, Ph.D.
Dr. Golden-Mason oversees the AMI Cores equipment and has extensive experience in a broad array of molecular and immunologic techniques.
Core Research Specialist: Heather Johnson, B.A., M.S.
Ms. Johnson provides training and assistance for all core users using the shared equipment in this core, and consults with Investigators and personnel about new projects/analyses. She is also trained in the use and operation of the Core‘s HPLC system and the shared equipment base. She provides all HPLC services as well as assisting in the Core’s extensive instrumentation base’s day-to-day uses, operation, maintenance, repair, safety, plus all record-keeping and reporting of usages.
Facilities and Resources
Location: 6th and 8th floor
Equipment:
- Bio-Rad CFX3984 Touch Real-Time PCR System (HMR 615)
- Bio-Rad ChemiDoc MP Imaging System (HMR 615)
- ABI/Life Technologies 7900HT Sequence Detection System for real time PCR + PC (HMR 615)
- ABI/Life Technologies StepOne Plus 96-well RT PCR system w/PC control/data acq. (HMR 615)
- ABI/Life Technologies Veriti Fast 96W Thermal Cycler w/PC control/data acquisition (HMR 615)
- LI-COR Odyssey 9120 Infrared Imaging System w/PC control/data acquisition (HMR 615)
- Thermo NanoDrop 8000 spectrophotometer w/PC control/data acquisition (HMR 615)
- BMG FLUOstar Omega microplate reader w/PC control/data acquisition (HMR 615)
- Thermo Labsystems Luminoskan Ascent plate reader w/PC control/data acquisition (HMR 615)
- HPLC system: Shimadzu with dual LC-20AT pumps, SIL-20AC autosampler and CBM-20A controller (HMR 615)
- Beckman LS 6000TA scintillation spectrometer (HMR 610)
- Sorvall RC-6+ superspeed centrifuge with SS-34, GSA, SLA 1500 & FiberLite F13-14x50cy rotors (HMR 610)
- Beckman L8-80M ultracentrifuge with 45Ti SW28 and SW41 rotors (HMR 610)
- Beckman Optima Max-E benchtop ultracentrifuge with TLA 100.3 rotor (HMR 610)
- Beckman Allegra 6R refrigerated benchtop centrifuge (HMR 610)
- Hoshizaki ice-maker (HMR 610a)
- Sanyo MDF-U71VC -86 degree freezer (HMR 805)
- Labnet HERMLE Z400K refrigerated benchtop centrifuge (HMR 804)
- Fisher Scientific model 500 Sonic Dismembrator (HMR 802)
- Millipore Milli-Q Advantage water purification system (HMR 614)
- VWR Symphony ULT -86 degree freezer (HMR 614)
- Seahorse Bioscience Model XF24 Extracellular Flux Analyzer (HMR 612)
- Kenmore -20 degree frost-free freezer (HMR 612)
- COY Incubator w/CO2 & O2 control (HMR 512)
To access and use the Core, contact Ms. Heather Johnson (heather.johnson@usc.edu)
Translational/BioRepository/Bioinformatics/Biostatistics (T3B) Core
Goals of the T3B Core:
- To provide statistical support to investigators as they plan and execute new studies involving human subjects through consultation about study design for human subjects and experimental research, advice and training on statistical applications and assistance with statistical analyses.
- Provide a biorepository, which is designed to collect, process, store and distribute biological specimens and promote scientific investigation.
- Provide bioinformatics support to RCLD members to assist in experimental design, bioinformatics analyses, data retrieval and management, storage, quality control, and write-up.
Director: Norah Terrault, M.D., M.P.H.
Dr. Terrault provides expert insights, oversight and support in the design and analysis of patient-based studies. She was recruited from UCSF where she had a long-standing role on the Executive Committee of their Liver Center, working closely with biostatisticians to develop research projects across a broad spectrum of liver diseases, including viral hepatitis, Non-Alcoholic SteatoHepatitis (NASH), alcoholic liver disease, and liver transplantation. She has she been able to recognize evolving areas of investigation with the opportunity for clinical studies as the practice of liver disease shifts. Her leadership aligns very well with the objectives of this new core.
Associate Director: Hugo R. Rosen, M.D.
Dr. Rosen oversees the biorespository of over 50,000 specimens from patients with liver disease linked to an electronic ACCESS database. He has formed a Department of Medicine-wide initiative to provide technical support and biorepository services.
Associate Director: Lucy Golden-Mason, Ph.D.
Dr. Golden-Mason manages the existing tissue bank (via a specifically designed ACCESS database) and will direct the Biorepository. She will be responsible for compliance with all federal regulatory regulations, quality control for banked specimens and implementation of tissue banking access and use. She will work with the Director and Associate Director to standardize all tissue collection, processing, storage and distribution; she also provides training and oversight to ensure rigorous experimental design for robust and unbiased results.
Assistant Director: Matthew Salomon, Ph.D.
Dr. Salomon provides a wide array of services including single cell transcriptome analyses, GWAS, genome-wide and targeted epigenetics studies.
Population Science Program:
Director: Wendy Setiawan, Ph.D.
The overarching goals of the Population Science Program are to identify, describe, and characterize risk factors for liver cancer and other liver diseases and to ultimately reduce disease burden and improve outcomes. We conduct observational studies on disease patterns, etiology, and outcomes, with emphasis on underserved and minority populations in the catchment area and racial/ethnic disparities.
Program research is focused on the following scientific themes: 1) descriptive epidemiology including studies of incidence and prevalence trends; 2) lifestyle, genetic, and environmental factors associated with disease incidence and progression; 3) biomarkers related to disease etiology and/or early detection; 4) risk modeling and stratification. Resources include population-based cohorts (e.g. Multiethnic Cohort Study), cancer registries (the Cancer Surveillance Program, SEER), and population-based Los Angeles Residual Tissue Repository.
For services, please fill out the attached RCLD Bio-Respository Request form and return to Lucy Mason-Golden, Ph.D. – lucy.golden@med.usc.edu.
*This core is supported by the USC Department of Medicine.*