(Originally posted at USC Stem Cell.)
By Cristy Lytal
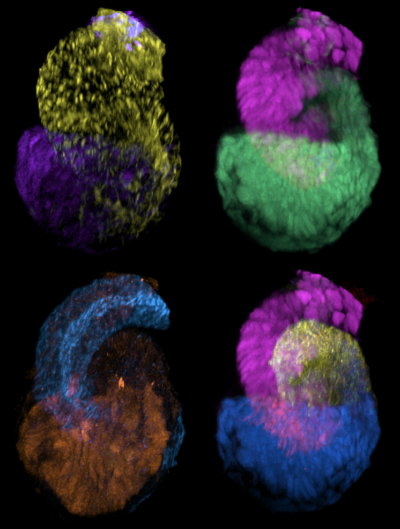
By superimposing images of several of the kidney’s filtering units, known as nephrons, researchers can visualize how little these structures deviate from a stereotypical developmental trajectory. (Image by Nils Lindstrom/McMahon Lab/USC Stem Cell)
When it comes to building a kidney, only nature possesses the complete set of blueprints. But a USC-led team of scientists has managed to borrow some of nature’s pages through a comprehensive analysis of how kidneys form their filtering units, known as nephrons.
Published in the journal Developmental Cell, the study from Andy McMahon’s lab in the Department of Stem Cell Biology and Regenerative Medicine at USC was led by Nils Lindström, who started the research as a postdoctoral fellow and is now an assistant professor in the same department. The study also brought in the expertise of collaborators from Princeton University and the University of Edinburgh in the UK.
The team traced the blueprints for how cells interact to lay the foundations of the human kidney, and how abnormal developmental processes could contribute to disease. Their findings are publicly available as part of the Human Nephrogenesis Atlas, which is a searchable database showing when and where genes are active in the developing human kidney, and predicting regulatory interactions going on in developing cell types.
“There’s only one way to build a kidney, and that’s nature’s way,” said McMahon, who is the director of the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC. “Only by understanding the logical framework of normal embryonic development can we improve our ability to synthesize cell types, model disease and ultimately build functional systems to replace defective kidneys.”
To reconstruct nature’s molecular and cellular blueprints, the team studied hundreds of human and mouse nephrons at various points along their typical developmental trajectories. This allowed the researchers to compare important processes that have been conserved during the nearly 200 million years of evolution since humans and mice diverged from their common mammalian ancestor.
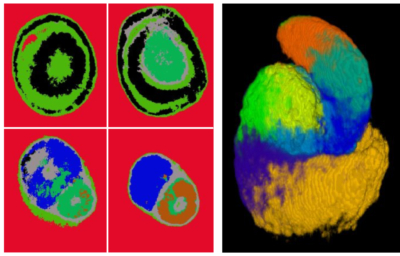
Machine learning of complex protein patterns predicts precursor cell populations in the developing nephron. The nephron is the filtering unit of the kidney. (Image by Nils Lindström/McMahon Lab/USC Stem Cell)
The study details the similar genetic machinery that underpins nephron formation in humans and mice, enabling other groups of scientists to follow the logic of these developmental programs to make new types of kidney cells. All told, there are at least 20 specialized cell types that form the kidney’s intricate tubular network, which helps maintain the body’s fluid and pH balance, filter the blood, and concentrate toxins into the urine for excretion.
“By generating detailed views of the beautifully complex process by which human nephrons form, we aim to enhance our understanding of development and disease, while guiding efforts to build synthetic kidney structures,” said Lindström.
The scientists were also able to determine the precise positions of expressed genes with known roles in Congenital Abnormalities of the Kidney and Urinary Tract (CAKUT). In specific types of cells, the researchers identified networks of interacting genes. Based on these associations, the team predicted new candidate genes to explore in CAKUT and other kidney diseases.
“Our approach of inferring spatial coordinates for genes expressed in individual cells could be widely used to create similar atlases of other developing organ systems—something that is an important focus of many research groups around the world,” said Lindström. “The study exemplifies the impact of collaborative science bringing together expertise across the U.S. and Europe to connect developmental anatomy with cutting-edge molecular, computational and microscopy tools.”
Additional co-authors are: Riana K. Parvez, Andrew Ransick, Guilherme De Sena Brandine, Jinjin Guo, Tracy Tran, Albert D. Kim, Brendan H. Grubbs, Matthew E. Thornton, Jill A. McMahon, Seth W. Ruffins, and Andrew D. Smith from USC; Rachel Sealfon, Xi Chen, and Jian Zhou from the Flatiron Institute and Princeton University; Alicja Tadych from Princeton University; Aaron Watters, Aaron Wong, and Elizabeth Lovero from the Flatiron Institute; Bill Hill from the University of Edinburgh; and Chris Armit the University of Edinburgh and BGI Hong Kong.
Fifty percent of the research was supported by federal funds from the National Institutes of Health (DK054364, DK110792, U24DK100845, UGDK114907, U2CDK114886, and UH3TR002158). Additional support came from the California Institute for Regenerative Medicine (LA1-06536), and the Genetic Networks program of the Canadian Institute for Advanced Research (CIFAR).