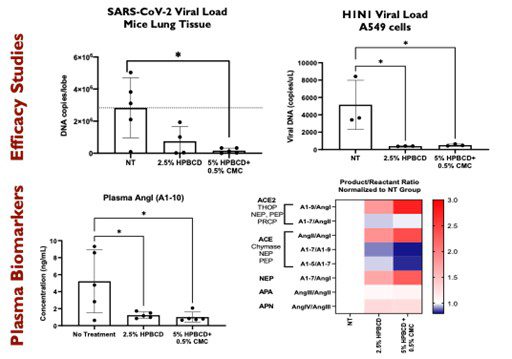
Figure 1 HPBCD barrier prevents infection of SARS-CoV-2 Delta infection in K18-hACE2 mice and H1N1 infection in A549 lung epithelial cell line. Plasma biomarkers (Angiotensin I and Angiotensin II) correlates with the efficacy response observed in these models.
Mucocutaneous membranes lining the eye and nasal cavity are susceptible sites of viral infections leading to conjunctivitis (pink eye) or respiratory infections whose total direct cost is estimated as $16 billion annually in the US. Traditional prevention approaches like vaccines and handwashing have not yielded much effectiveness due to poor compliance and emergence of variants. There is an urgent need to develop novel, accessible, easily administered, and effective mucous membrane-augmenting barrier that will be agnostic to all viral variants and protect the public from seasonal viruses and other pandemic-causing viruses like the coronaviruses.
To address this challenge, our interdisciplinary team led by Dr. Isaac Asante has been developing a novel eye drop and nasal spray that can speedily translate to clinic, providing a tremendous impact on preventing eye and respiratory infections from seasonal viruses. The group is repurposing a safe and FDA-approved pharmaceutical excipient, hydroxypropyl betacyclodextrin (HPBCD) for the development of this intervention.
Dr. Asante’s group recently showed the HPBCD nanobarrier prevents infection of lentiviruses, SARS-CoV-2 pseudotype variants, influenza viruses and SARS-CoV-2 viruses delta variant in in vitro models as well as SARS-CoV-2 viruses in mice models (Figure 1). This is an innovative and prophylactic intervention that will the first agnostic topical and physical barrier that will be noninvasive, widely accessible, easily administered, and acutely used to prevent eye and respiratory seasonal viral infections during pandemics and in densely populated / high-risk environments.
Dr. Asante’s team is one of the few leaders in this field of utilizing metabolomics to guide the development of therapeutic interventions in ophthalmology. If the ongoing efforts of Dr. Asante’s team are successful, this intervention will impact several patients who suffer from pink eye and other respiratory viral infections.