By Sidney Taiko Sheehan
Just last year, Danny JJ Wang, PhD, director of imaging technology innovation at the USC Mark and Mary Stevens Neuroimaging and Informatics Institute (USC INI) and his team at the start-up company Hura Imaging, Inc., received a two-year, $1.6 million grant from the National Institutes of Health’s Small Business Innovation Research (SBIR). The SBIR program supports the translation of technologies from the lab to commercial use through small business innovation and commercialization.
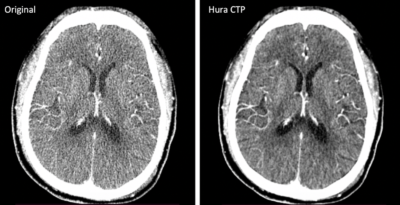
Compared to existing low-dose CT technologies, the research team’s KWIA technique has the advantages of fast computation speed and excellent retention of the texture and resolution of CT images. (Photo/Courtesy of Danny JJ Wang)
Wang and his team are addressing the growing concern that radiation involved in computed tomography (CT) scans can be unnecessarily harmful to patients. CT scans can certainly save lives by catching abnormalities, but there is a strong desire for methods that can lower the risk of cancer with reduced use of radiation. However, low dose CT scans result in low contrast or noisy images.
“Our technology reduces the image noise of CT perfusion and improves image contrast by about 50-75% without compromising imaging speed,” says Wang. “Compared to existing low-dose CT technologies, our technique has the advantages of fast computation speed and excellent retention of the texture and resolution of CT images. We are delighted to report that we have now received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for our CT perfusion software (Hura CTPTM).”
“Dr. Wang’s technique dramatically improves the quality and signal-to-noise ratio of CT perfusion images, with minimal additional computation time,” says Vinay Duddalwar, MD, professor of clinical radiology at the Keck School of Medicine of USC. “The technology may impact the safety and clinical practice of CT perfusion.” Dr. Duddalwar is the lead investigator of the SBIR study at the Keck School.
FDA 510(k) clearance is a critical and necessary step when preparing a “device” for market in the U.S. “Dr. Wang set out with the goal of leading the INI’s efforts to use our computational resources and expertise in clinical imaging to make CT scans safer while improving image quality,” says Arthur W. Toga, PhD, provost professor of ophthalmology, neurology, psychiatry and the behavioral sciences, radiology and engineering, the Ghada Irani chair in neuroscience at the Keck School, and director of the INI. “Dr. Wang is now one step closer to commercializing this work so that it can be widely used in clinical practice to help keep patients safe while improving the quality of clinical care.”
The INI is collaborating with USC’s radiology department and Hura Imaging. The INI provides the facilities and computational resources through its Laboratory of Neuro Imaging (LONI) and Hura Imaging is handling commercialization of the technology. “I’m grateful for the opportunity to impact translational research and to celebrate this milestone with my colleagues,” says Wang.
