Excess cholesterol accelerates damage of fatty liver disease, according to Keck School of Medicine of USC research
By Wayne Lewis
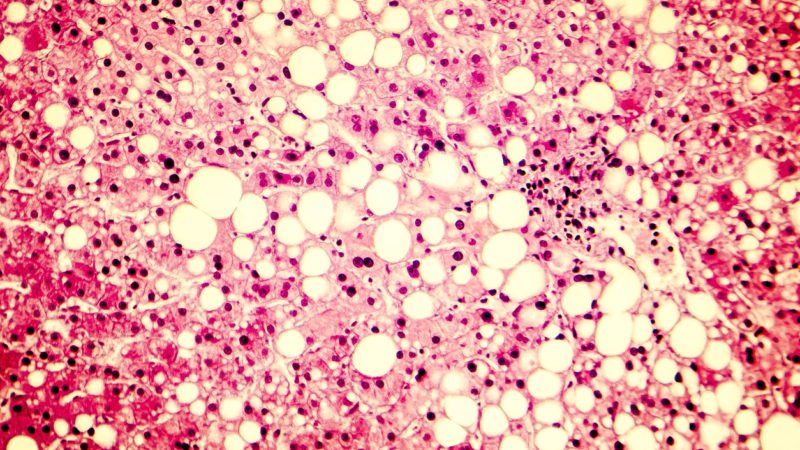
Image of fatty liver disease showing triglyceride fat accumulated inside liver cells. (Image iStock)
There’s a long-established link between a high-fat, high-sugar diet and fatty liver disease, which can lead to life-threatening conditions such as cirrhosis and liver cancer. Now, new research from the Keck School of Medicine of USC adds some detail and dimension to this picture.
The lab study, published in Frontiers in Immunology, is the first-ever to focus on how different amounts of cholesterol as part of a diet high in fat and sugar affect fatty liver disease progression. Modeling the disease in mice, the investigators demonstrated that high cholesterol intake can make fatty liver disease worse — driving inflammation and scarring — and that, importantly, scar tissue can persist even after switching to a diet low in cholesterol. The findings also indicated that a high-cholesterol diet can create long-lasting dysfunction in a specific population of immune cells previously shown to play a role in fatty liver disease.
“We saw that you may have a high-fat and high-sugar diet, but when you add high cholesterol to that, it will accelerate the process that causes inflammation in your liver,” said corresponding author Ana Maretti-Mira, PhD, an assistant research professor of medicine at USC. “People focus on high cholesterol as a risk for heart disease, but we showed that your liver may also be affected, causing inflammation, scarring and, potentially, cirrhosis.”
High cholesterol makes fatty liver disease worse
The researchers fed mice a high-fat, high-sugar diet shown to cause a form of advanced fatty liver disease similar to human illness.
The mice were split into three groups that received different amounts of cholesterol in their food for 20 weeks — midlife for the animals. The low-cholesterol group received one-quarter the cholesterol compared to medium; the high-cholesterol group received 25 times more than the low-cholesterol group.
After 20 weeks, the livers of mice from all three groups showed accumulation of fat, a benign feature of fatty liver disease, but the high-cholesterol group had more advanced disease, with increased inflammation and scar tissue.
For the following 10 weeks, mice from all three groups received low cholesterol as part of a diet that remained high in fat and sugar. At the end of that time, that change in diet had reversed inflammation in the original high-cholesterol mice, but had not reduced scar tissue. This finding shows that damage caused by high cholesterol can be hard to undo.
Damaging alterations to multipurpose cells in the body’s first line of defense
Maretti-Mira and her colleagues also delved into changes in cells called macrophages, which are important to the innate immune response — the front line of the body’s natural defenses. There are multiple roles for macrophages. They can spur inflammation to fight off invading bugs; quell inflammation when danger has passed; communicate with other immune cells; and even help with healing.
Macrophages are involved in fatty liver disease, but in a paradoxical way: They seem to help drive damage, but the damage doesn’t heal well without them.
The researchers used RNA sequencing to contrast which genes were activated in liver macrophages from mice in the study with gene expression in normal macrophages. In line with the team’s other findings, genes that cause inflammation and scarring were turned on by the medium- and high-cholesterol diets, while genes associated with healing were activated by the switch to low cholesterol intake.
However, in macrophages from the original high-cholesterol group, genes that lead to scarring remained active even after the switch. This key result suggests that regularly eating too much cholesterol can cause long-term, difficult-to-reverse changes in macrophages, sabotaging their healing action so they continue to cause liver damage.
What this suggests for your diet: everything in balance
The high-fat, high-sugar diet given to mice in the study has unfortunate similarities to the typical Western diet in humans.
“Our daily diet has lots of carbohydrates, such as sugary drinks, bread, rice and pasta,” Maretti-Mira said. “Then there’s high fat, since everybody likes deep fried foods. At the same time, we don’t have the same active life we used to, so we end up eating much more than our body needs.”
Nonetheless, she emphasizes that her team’s findings do not suggest that people should completely cut cholesterol from their diets. After all, a certain amount of fat, including cholesterol, is needed for our bodies to function properly.
Rather, moderation is key.
“Everything’s a balance,” Maretti-Mira said. “If what — and how much — you eat is causing this excessive inflammation in your liver, it’s time to take care of yourself. Change your diet and exercise more, so you can burn that fat in the liver, because it can cause damage in the long term.”
About the study
Other co-authors of the study are Matthew Salomon, Angela Hsu, Gary Kanel and Lucy Golden-Mason, all of USC.
The study was supported by the National Institutes of Health (DK117004, DK106491) and the USC Research Center for Liver Disease (P30DK048522).