Megan McCain, Giorgia Quadrato and Leonardo Morsut have been awarded a four year National Science Foundation grant to develop better organoids, to help us understand human brain development and disease.
(Originally posted at Viterbi.)
The human brain is an incredibly complex organ to study in its living tissue form. Researchers cannot experiment on human tissue directly, and animal models are often too different to human physiology to be effective.
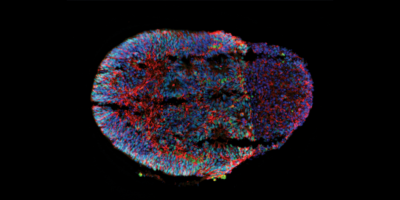
For this reason, in the last decade, neurological research has been increasingly turning to “brain-on-a-chip” organoid models to give researchers living examples to demonstrate brain development, and how to effectively treat brain diseases and disorders. Organoids are grown out of stem cells into new cell clusters that mimic the structure of and features of a whole organ, such as the brain.
Co-principal investigator Megan McCain from the USC Viterbi Department of Biomedical Engineering will partner with fellow co-principal investigators Giorgia Quadrato and Leonardo Morsut in the Department of Stem Cell Biology and Regenerative Medicine at the Keck School of Medicine of USC on a four year, $1.5m NSF-funded project to vastly improve the process of developing brain organoids. The project aims to make the end products more consistent and reliable as tools for brain researchers.
McCain is the Chonette Early Career Chair and assistant professor of biomedical engineering at USC Viterbi. She said that one of the biggest hurdles in the current process of organoid creation was their lack of uniformity.
“Researchers start with a small group of human stem cells, and then give them some chemical cues to direct their development into brain tissue, but ultimately, the cells are mostly left to their own devices, so they often grow very randomly,” McCain said. “They divide and differentiate into other cell types in a somewhat haphazard process. So if you make ten organoids, all ten of them will look slightly different.”
McCain said it was this issue that could be detrimental to the accuracy of using organoids in certain types of research, such as the testing of therapeutics and how the brain responds to these drugs, and that organoids needed to be more uniform and reproducible in order to be more effective tools.
“Drug testing with organoids today is very challenging because it is hard to separate the effect of the drug from the inherent variability of the organoids themselves,” McCain said.
Morsut said that his part of the project involves developing synthetic molecular tools to simplify the analysis of what happens during the formation of brain organoids in a laboratory setting.
“The normal molecules that are used by the cells to self-organize, as well as to make decisions, are linked in very complex networks, and we need artificial tools to tease apart the contributions of these different components,” Morsut said. “The challenge—and the exciting part—is to use these tools to explain the remarkable phenomenon of self-organization.”
McCain said her lab will focus on the device side—the organ-on-chip—and make microfluidic components for growing and studying organoids under more defined conditions.
“This will likely improve reproducibility and possibly organoid maturity, which is another major bottleneck,” McCain said.
In order to do this, McCain’s lab will be repurposing a microfluidic device that they previously used to help explanted zebrafish hearts to remain alive longer and regenerate, while also live-imaging the process.
The device, when applied to brain organoids, will constrain the cells in chambers where the team can run experiments through them in a more controlled way, to see if this improves the consistency of the end product.
“And we will also be able to image and monitor their reproducibility by putting them in this little device where they’re all growing in the same configuration,” McCain said.
Quadrato, an expert in brain organoids, said she was excited about the collaboration, because a multidisciplinary approach was needed to improve the relevance of current organoids.
“One way to improve brain organoid-to-organoid reproducibility is to expose them to small molecules to direct differentiation of the stem cells,” Quadrato said.
She said that these small molecules unfortunately sometimes have problematic side effects, such as impairing the survival of other non-neural cell types, or skewing tests of potential treatments for brain diseases and disorders.
“In our proposal, we suggest an alternative strategy to increase brain organoid reproducibility that does not cause these side effects, and therefore can be used to create organoids to accurately model disease,” Quadrato said.